Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Momma, Yuichiro*; Sakairi, Masatoshi*; Ueno, Fumiyoshi; Otani, Kyohei
Zairyo To Kankyo, 71(5), p.133 - 137, 2022/05
The effect of the corrosion inhibitor on the corrosion of steel under a thin solution layer was investigated. As a result of forming a thin solution layer with a thickness of 1.0-0.2 mm on the specimen, adding a mixed solution of sodium molybdate and aluminum lactate as a corrosion inhibitor, and performing electrochemical measurement, the corrosion inhibitor suppresses the anodic reaction. And in the thin solution layer, it was suggested that the morphology of the protective layer structure by the corrosion inhibitor changed according to the amount of liquid as compared with the bulk immersion.
Soma, Yasutaka; Kato, Chiaki
Zairyo To Kankyo 2022 Koenshu (CD-ROM), p.219 - 220, 2022/05
It is important to understand the electrochemical properties of stainless steel in environment created within crevice of stainless steel in high temperature water (crevice environment). This is because acidification and concentration of impurity ions occur in the crevice environment and this is common inside the stress corrosion crack. In this study, we reproduced the crevice environment in bulk scale and investigated mainly the effect of Cr concentration on the electrochemical properties of Fe-Cr-Ni alloys. Polarization curves of Fe-20Ni-xCr (x=16.4, 23, 26) were measured in water with a temperature of 288 C, a Cl concentration of 2
C, a Cl concentration of 2 10
10 mol/dm
mol/dm , a pH value of about 4.5, and a dissolved hydrogen concentration of 10 ppb. The peak currents of active dissolution (at -400 mV) and passive current density (at -50 mV) for specimens with Cr concentrations x = 16.4, 23, and 26% were approximately 13.8, 15.9, 10.0
, a pH value of about 4.5, and a dissolved hydrogen concentration of 10 ppb. The peak currents of active dissolution (at -400 mV) and passive current density (at -50 mV) for specimens with Cr concentrations x = 16.4, 23, and 26% were approximately 13.8, 15.9, 10.0  Acm
Acm , and 18.4, 8.5, 8.5
, and 18.4, 8.5, 8.5  Acm
Acm , respectively. Although the current values of x=26 were slightly lower in both cases, it was concluded that there was no clear dependence of the polarization curve on Cr concentration in this environment.
, respectively. Although the current values of x=26 were slightly lower in both cases, it was concluded that there was no clear dependence of the polarization curve on Cr concentration in this environment.
 Sr dissolved solution on corrosion potential of type 316L stainless steel
Sr dissolved solution on corrosion potential of type 316L stainless steelAoyama, Takahito; Kato, Chiaki; Sato, Tomonori; Sano, Naruto; Yamashita, Naoki; Ueno, Fumiyoshi
Zairyo To Kankyo, 71(4), p.110 - 115, 2022/04
no abstracts in English
Momma, Yuichiro*; Sakairi, Masatoshi*; Ueno, Fumiyoshi; Otani, Kyohei
Zairyo To Kankyo, 71(4), p.121 - 125, 2022/04
The effect of solution layer thickness on the atmospheric corrosion of carbon steel was investigated using novel devices fabricated by a 3D printer. These novel devices allowed us to control the solution layer thickness precisely. Potentiodynamic polarization measurements were performed under thickness-controlled solution layer, and oxygen diffusion limiting current density ( ) and anodic current density (
) and anodic current density ( ) were measured. As the solution layer become thinner,
) were measured. As the solution layer become thinner,  increased and
increased and  decreased. This result indicates that corrosion accelerates when the solution layer becomes thinner. The diffusion coefficient of oxygen was calculated as 3.20
decreased. This result indicates that corrosion accelerates when the solution layer becomes thinner. The diffusion coefficient of oxygen was calculated as 3.20 10
10 cm
cm s
s from the relationship between
from the relationship between  and solution layer thickness, and the critical diffusion thickness was estimated to be 0.87 mm.
and solution layer thickness, and the critical diffusion thickness was estimated to be 0.87 mm.
 Np
NpIrisawa, Eriko; Kato, Chiaki; Yamashita, Naoki; Sano, Naruto
Zairyo To Kankyo, 71(3), p.70 - 74, 2022/03
In order to evaluate the corrosion of stainless steels used in spent nuclear fuel reprocessing facilities, the immersion corrosion tests and polarization measurements were performed using R-SUS304ULC stainless steel in nitric acid solution containing a kind of radionuclides,  Np. At temperatures above 328 K, the corrosion potential was higher than that in nitric acid solution and was near the transpassive region. From the comparison between the corrosion amount calculated by the immersion corrosion tests and the polarization resistance, the values of
Np. At temperatures above 328 K, the corrosion potential was higher than that in nitric acid solution and was near the transpassive region. From the comparison between the corrosion amount calculated by the immersion corrosion tests and the polarization resistance, the values of 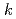 =0.018-0.025 V were obtained as a conversion factor, and the possibility of calculating the corrosion amount from the electrochemical measurement was examined.
=0.018-0.025 V were obtained as a conversion factor, and the possibility of calculating the corrosion amount from the electrochemical measurement was examined.
Otani, Kyohei; Ueno, Fumiyoshi; Kato, Chiaki
Zairyo To Kankyo, 71(2), p.40 - 45, 2022/02
The purpose of this study is to investigate the effect of oxygen concentration in the air on the corrosion rate of carbon steel in an air/solution alternating environment in the low oxygen concentration range and to clarify the corrosion rate and corrosion mechanism of carbon steel depending on the oxygen concentration in air by the mass change of specimens before and after the corrosion test and observing the iron rust layer formed on the surface of carbon steel. The corrosion rate increases with increasing oxygen concentration in the air, and the gradient of the corrosion rate decreases gradually. The maximum erosion depth increased with increasing oxygen concentration except for the case of 1% oxygen concentration, however, the maximum erosion depth for 1% oxygen concentration was larger than that for 5% air oxygen concentration.
Otani, Kyohei; Kato, Chiaki
Zairyo To Kankyo, 70(12), p.480 - 486, 2021/12
This is a comprehensive paper of the corrosion of carbon steel in air/solution alternating condition. From cross-sectional observation and analysis of the iron rust layer formed on the surface of carbon steel in the alternating condition, it was found that a multilayered iron rust layer composed of red rust layer ( -FeOOH), rust crust layer (Fe
-FeOOH), rust crust layer (Fe O
O ), inner crystal (Fe
), inner crystal (Fe O
O ), and inner rust layer was formed on carbon steel. The multi-layered iron rust layer would accelerate the cathodic oxygen reduction reaction, and the reason why the corrosion rate of the carbon steel in the alternating condition was accelerated. The effect of artificial seawater (ASW) composition on the corrosion rate of carbon steel in air/solution alternating condition was investigated. It was found that the corrosion rate increased with increasing concentration from pure water to 200 times diluted ASW, and decreased with increasing concentration from 20 times diluted ASW to no diluted ASW. The Mg and Ca ions in ASW precipitated on the reaction interface and formed a metal cation layer, which inhibited the oxygen reduction reaction, and thus the corrosion of carbon steel was inhibited in the highly concentrated ASW.
), and inner rust layer was formed on carbon steel. The multi-layered iron rust layer would accelerate the cathodic oxygen reduction reaction, and the reason why the corrosion rate of the carbon steel in the alternating condition was accelerated. The effect of artificial seawater (ASW) composition on the corrosion rate of carbon steel in air/solution alternating condition was investigated. It was found that the corrosion rate increased with increasing concentration from pure water to 200 times diluted ASW, and decreased with increasing concentration from 20 times diluted ASW to no diluted ASW. The Mg and Ca ions in ASW precipitated on the reaction interface and formed a metal cation layer, which inhibited the oxygen reduction reaction, and thus the corrosion of carbon steel was inhibited in the highly concentrated ASW.
Hata, Kuniki
Zairyo To Kankyo, 70(12), p.468 - 473, 2021/12
In order to estimate corrosive environment in the contaminated water at Fukushima Daiichi Nuclear Power Station, effects of oxidants, such as H O
O , which were generated from water radiolysis, should be taken into account due to the irradiation field in the reactor building. The process of water radiolysis and the amounts of these oxidants can change depending on the conditions of water and types of radiation. After the accident, a variety of factors, which can affect water radiolysis, such as seawater constituents, surface of oxides, and
, which were generated from water radiolysis, should be taken into account due to the irradiation field in the reactor building. The process of water radiolysis and the amounts of these oxidants can change depending on the conditions of water and types of radiation. After the accident, a variety of factors, which can affect water radiolysis, such as seawater constituents, surface of oxides, and  -radionuclides, had been discussed. In this paper, these effects on radiolysis are reviewed for the better understanding of the corrosive environment in the contaminated water.
-radionuclides, had been discussed. In this paper, these effects on radiolysis are reviewed for the better understanding of the corrosive environment in the contaminated water.
Kato, Chiaki; Yamagishi, Isao; Sato, Tomonori; Yamamoto, Masahiro*
Zairyo To Kankyo, 70(12), p.441 - 447, 2021/12
Zeolite particles have been used in a Cs adsorption vessel for purification of contaminated water in Fukushima Dai-ich Nuclear Power Station (1F). The used Cs adsorption vessels were kept in storage space on 1F site. The risk of localized corrosion of stainless steel used in the vessel was worried. To evaluate the risk of localized corrosion, using specially designed electrochemical testing apparatus was used under gamma-ray irradiation test. And, real size mock-up test conducted. The results showed the potential change caused by creation of H O
O by water radiolysis decreased by zeolite particles and the enrichment of chloride ion concentration in the vessel do not propagate during dry up procedure of Cs adsorption vessel. These data indicate the risk of localized corrosion of Cs adsorption vessel may stay at considerably low level.
by water radiolysis decreased by zeolite particles and the enrichment of chloride ion concentration in the vessel do not propagate during dry up procedure of Cs adsorption vessel. These data indicate the risk of localized corrosion of Cs adsorption vessel may stay at considerably low level.
Wakai, Satoshi*; Hirano, Shinichi*; Ueno, Fumiyoshi; Okamoto, Akihiro*
Zairyo To Kankyo, 70(12), p.491 - 496, 2021/12
After Fukushima Daiichi Nuclear Power Station accident, various corrosion mitigating activities have been treated, and severe corrosion incident have never taken placed. On the other hand, the facilities were exposed sea water, and some of them have continuously exposed to ground water. The exposure of metal materials to environmental water has a risk of microbiologically influenced corrosion (MIC). In this paper, we summarize the latest knowledge of MIC and the task of MIC in the decommissioning of Fukushima Daiichi Nuclear Power Station.
Soma, Yasutaka; Kato, Chiaki
Dai-68-Kai Zairyo To Kankyo Toronkai Koenshu (CD-ROM), p.205 - 206, 2021/10
This study investigates the effect of temperature on dissipation behavior of Cl ion within the crevice of stainless steel. Concentration of Cl ion was evaluated by conductivity measured by using sensors installed at crevice specimen. At 50 and 80  C, Cl ions within the crevice of PEEK and Pt dissipated in accordance with concentration diffusion. On the contrary, dissipation speed of Cl ions inside the Type-304L stainless steel were much lower than those anticipated by simple concentration diffusion. This behavior attribute to the anodic dissolution of stainless steel inside the crevice, therefore, to quantitatively understand the effect of temperature on the dissipation behavior, it is necessary to know the anodic dissolution rate and occurrence of localized corrosion. Numerical analysis taking the effect of concentration diffusion and migration into account is also needed.
C, Cl ions within the crevice of PEEK and Pt dissipated in accordance with concentration diffusion. On the contrary, dissipation speed of Cl ions inside the Type-304L stainless steel were much lower than those anticipated by simple concentration diffusion. This behavior attribute to the anodic dissolution of stainless steel inside the crevice, therefore, to quantitatively understand the effect of temperature on the dissipation behavior, it is necessary to know the anodic dissolution rate and occurrence of localized corrosion. Numerical analysis taking the effect of concentration diffusion and migration into account is also needed.
Ishijima, Yasuhiro; Ueno, Fumiyoshi; Abe, Hitoshi
Zairyo To Kankyo, 70(6), p.192 - 198, 2021/06
The time dependence of corrosion behavior on tantalum used in nuclear fuel reprocessing equipment in sodium hydroxide solution was investigated by immersion corrosion tests, and the mechanism of aging change was discussed from surface observations and electrochemical measurements. The immersion tests were carried out at room temperature with NaOH concentrations ranging from 1 to 7 mol/L and immersion times ranging from 24 to 168 hr, respectively. The corrosion rate increased with NaOH concentration, but peaked with immersion time and then decreased. The time to peak of corrosion rate was shorter with higher NaOH concentration. The SEM observations and Raman analysis at the surface of the specimens that were cleaned and weighed after the immersion test did not show any film formation. On the other hand, the polarization resistance showed a constant value or an increase after a decrease immediately after immersion. It is suggested that the change in corrosion rate is affected by the formation of film by immersion, since the value of polarization resistance is almost the same as the sum of film resistance and charge transfer resistance. The film was considered to be mainly Na Ta
Ta O
O formed by the dissolution of Ta.
formed by the dissolution of Ta.
Hirota, Noriaki; Takeda, Kiyoko*; Tachibana, Yukio; Masaki, Yasuhiro*
Zairyo To Kankyo, 70(3), p.68 - 76, 2021/03
Corrosion resistance of stainless steels and Ni-based alloys were evaluated in a sulfuric acid decomposition gas at high temperature. The evaluation were carried out in an environment simulated in the sulfuric acid decomposition reaction vessel for thermochemical hydrogen production process (IS process). Their corrosion films were also analyzed for better understanding of the corrosion behavior. As a result, after 100 hour corrosion test, Ni-based alloy containing 2.4% Si showed good corrosion resistance. Ferritic stainless steel containing 3% Al (3Al-Ferrite) showed better corrosion resistance. Its corrosion rate was lower than that of SiC (0.1mm/year), which is a candidate material for the sulfuric acid decomposition reaction vessel. On the other hand, Ni-based alloy pre-filmed with Al O
O is prepared as the relative corrosion film of 3Al-Ferrite. Its corrosion rate was significantly higher than that of 3Al-Ferrite. As the result of EPMA analysis of these oxide films, Ni-based alloy containing 2.4% Si formed Si oxide film which had some cracks after the long term corrosion test. Therefore S penetrated into grain boundaries of the matrix through the oxide film. 3Al-Ferrite formed a thin and uniform Al
is prepared as the relative corrosion film of 3Al-Ferrite. Its corrosion rate was significantly higher than that of 3Al-Ferrite. As the result of EPMA analysis of these oxide films, Ni-based alloy containing 2.4% Si formed Si oxide film which had some cracks after the long term corrosion test. Therefore S penetrated into grain boundaries of the matrix through the oxide film. 3Al-Ferrite formed a thin and uniform Al O
O film, and the penetration of S into the grain boundaries was not observed. Al
film, and the penetration of S into the grain boundaries was not observed. Al O
O pre-film of Ni-based alloy also showed S penetration in the matrix because the Al
pre-film of Ni-based alloy also showed S penetration in the matrix because the Al O
O pre-film had many small defects originally. The corrosion oxide film of 3Al-Ferrite consisted of only
pre-film had many small defects originally. The corrosion oxide film of 3Al-Ferrite consisted of only  -Al
-Al O
O , while the Al
, while the Al O
O pre-film consist of
pre-film consist of  -Al
-Al O
O and
and  -Al
-Al O
O . Those results suggest that the better corrosion resistance of 3Al-Ferrite is due to the uniform formation of dense
. Those results suggest that the better corrosion resistance of 3Al-Ferrite is due to the uniform formation of dense  -Al
-Al O
O film at the early stage of the corrosion.
film at the early stage of the corrosion.
Otani, Kyohei; Tsukada, Takashi; Ueno, Fumiyoshi; Kato, Chiaki
Zairyo To Kankyo, 69(9), p.246 - 252, 2020/09
The purpose of this study was to investigate the effect of artificial sea water concentration on the corrosion rate of carbon steel under air/solution alternating condition, and to clarify the corrosion mechanism of carbon steel that changes with artificial seawater concentration. Mass measurements showed that the corrosion rate of carbon steel in the alternating condition accelerates with increasing concentration in the concentration region between deionized water to 200 times diluted artificial seawater (ASW), and the corrosion rate decreases with increasing concentration in the concentration region between 20 times diluted ASW to undiluted ASW. It can be considered that the reason why the carbon steel corrosion was suppressed in highly concentrated artificial seawater would Mg ions and Ca ions in the artificial seawater precipitate and cover on the surface due to the increase in pH near the surface by oxygen reduction reaction.
Yamamoto, Masahiro
Zairyo To Kankyo 2020 Koenshu (CD-ROM), p.9 - 16, 2020/05
The author has been continuing research and development for corrosion science for about forty years. One of the main targets of his research is applying computational science techniques on corrosion problems. The results are briefly introduced in this article. Also, the author organized some workshop for corrosion problems of 1F decommissioning procedure for several years. Such activities are evaluated for receiving the society award in JSCE.
Omori, Atsushi*; Akiyama, Eiji*; Abe, Hiroshi*; Hata, Kuniki; Sato, Tomonori; Kaji, Yoshiyuki; Inoue, Hiroyuki*; Taguchi, Mitsumasa*; Seito, Hajime*; Tada, Eiji*; et al.
Zairyo To Kankyo, 69(4), p.107 - 111, 2020/04
To evaluate the effect of oxidants, which are formed by radiolysis of water under gamma ray irradiation, on the corrosion of a carbon steel in humid environment, ozone was introduced as a model oxidant in to humidity-controlled air at 50 C in a thermo-hygrostat chamber. Corrosion monitoring was performed by using an Atmospheric Corrosion Monitor-type (ACM) sensor consisting of a carbon steel anode and an Ag cathode. The output current of the ACM sensor was increased with the increase in relative humidity and it was obviously increased with the increase in the introduced ozone concentration at each relative humidity. The results indicate that ozone accelerates the corrosion of the carbon steel. The effect of ozone on the corrosion acceleration is attributed to the fast reduction reaction and fast dissolution reaction in to water compared to that of oxygen.
C in a thermo-hygrostat chamber. Corrosion monitoring was performed by using an Atmospheric Corrosion Monitor-type (ACM) sensor consisting of a carbon steel anode and an Ag cathode. The output current of the ACM sensor was increased with the increase in relative humidity and it was obviously increased with the increase in the introduced ozone concentration at each relative humidity. The results indicate that ozone accelerates the corrosion of the carbon steel. The effect of ozone on the corrosion acceleration is attributed to the fast reduction reaction and fast dissolution reaction in to water compared to that of oxygen.
Kasahara, Shigeki; Chimi, Yasuhiro; Hata, Kuniki; Hanawa, Satoshi
Zairyo To Kankyo, 68(9), p.240 - 247, 2019/09
In order to study environment assisted cracking mechanism of stainless steel under BWR primary coolant condition, effects of applied load on oxidation in the vicinity of crack tips of CT specimens were evaluated. Loaded CT specimens were immersed in an aqueous condition at 290 C as a simulated BWR coolant condition, and microstructural observation on oxide near the tips of pre-cracks was carried out. Oxide inner layers, which consisted of fine grain magnetite containing Fe and Cr were formed, and oxide outer layers consisting of large grains of Fe
C as a simulated BWR coolant condition, and microstructural observation on oxide near the tips of pre-cracks was carried out. Oxide inner layers, which consisted of fine grain magnetite containing Fe and Cr were formed, and oxide outer layers consisting of large grains of Fe O
O were observed to cover the inner layers. FEM analysis of stress and strain in the loaded CT specimen suggests that both of dislocations due to localized plastic deformation and elastic strain could play important roles to accelerate inner oxide formation in the vicinity of the crack tip of the specimens.
were observed to cover the inner layers. FEM analysis of stress and strain in the loaded CT specimen suggests that both of dislocations due to localized plastic deformation and elastic strain could play important roles to accelerate inner oxide formation in the vicinity of the crack tip of the specimens.
Otani, Kyohei; Tsukada, Takashi; Ueno, Fumiyoshi
Zairyo To Kankyo, 68(8), p.205 - 211, 2019/08
In the present study, the iron rust layer formed on the low ally steel in air-solution alternating condition was investigated by cross-sectional observation and analysis, and the mechanism of accelerated corrosion of the steel in the alternating condition was clarified. Observation and analysis showed that the multi-layered iron rust layer composed of red rust layer (FeOOH), rust crust layer (Fe O
O ), inner crystal (Fe
), inner crystal (Fe O
O ), and inner rust layer was formed on the low alloy steel. It can be considered that the multi-layered iron rust layer accelerated the cathodic reaction rate of the steel in the alternating condition. This acceleration would be the reason why the corrosion rate of the low alloy steel in the alternating condition was accelerated.
), and inner rust layer was formed on the low alloy steel. It can be considered that the multi-layered iron rust layer accelerated the cathodic reaction rate of the steel in the alternating condition. This acceleration would be the reason why the corrosion rate of the low alloy steel in the alternating condition was accelerated.
Hirota, Noriaki; Kasahara, Seiji; Iwatsuki, Jin; Imai, Yoshiyuki; Ohashi, Hirofumi; Yan, X.; Tachibana, Yukio
Zairyo To Kankyo, 68(6), p.137 - 142, 2019/06
New corrosion test equipment for high temperature gas of decomposed sulfuric acid was manufactured in order to ascertain flow rate of sulfuric acid in the piping, occurrence of sulfuric acid decomposition reaction in the equipment, and temperature distribution inside the furnace tube. The flow rate of the sulfuric acid solution was constantly measured using an ultrasonic flowmeter. The SO concentration at the inlet of the test equipment was almost the same as that at the inlet of the sulfuric acid decomposer in the hydrogen production plant assuming a high-temperature gas cooled reactor hydrogen-power cogeneration system (GTHTR300C). On the other hand, during a test, leakage of sulfuric acid occurred from the fitting part at the outlet side. Hence the temperature distribution of the fitting part at the outlet side was investigated using fluid analysis. As a result, it was found that the temperature at the fitting was low enough to use fluorine joint grease when the distance was 0.05 m or more away from the outlet side pipe. An improved furnace tube was manufactured and the temperature was measured again at fitting part of the outlet side. The temperature was lower that the temperature limit of the joint grease and almost the same as the temperature distribution in the fluid analysis, and leakage of sulfuric acid has not occurred to date.
concentration at the inlet of the test equipment was almost the same as that at the inlet of the sulfuric acid decomposer in the hydrogen production plant assuming a high-temperature gas cooled reactor hydrogen-power cogeneration system (GTHTR300C). On the other hand, during a test, leakage of sulfuric acid occurred from the fitting part at the outlet side. Hence the temperature distribution of the fitting part at the outlet side was investigated using fluid analysis. As a result, it was found that the temperature at the fitting was low enough to use fluorine joint grease when the distance was 0.05 m or more away from the outlet side pipe. An improved furnace tube was manufactured and the temperature was measured again at fitting part of the outlet side. The temperature was lower that the temperature limit of the joint grease and almost the same as the temperature distribution in the fluid analysis, and leakage of sulfuric acid has not occurred to date.
Ueno, Fumiyoshi
Zairyo To Kankyo, 68(1), p.2 - 8, 2019/01
It is important to control the cooling water of light water reactors (boiling water reactor and pressurized water reactor) to suitable quality in order to reduce corrosion of structural materials and generation of radioactive corrosion products. For that purpose, monitoring of water quality using electrochemical measurement method is necessary. In this article, the application of ECP measurement to BWR is mainly focused, I describe the water quality of light water reactors and the necessity of electrochemical measurement.